Tracking Meterics
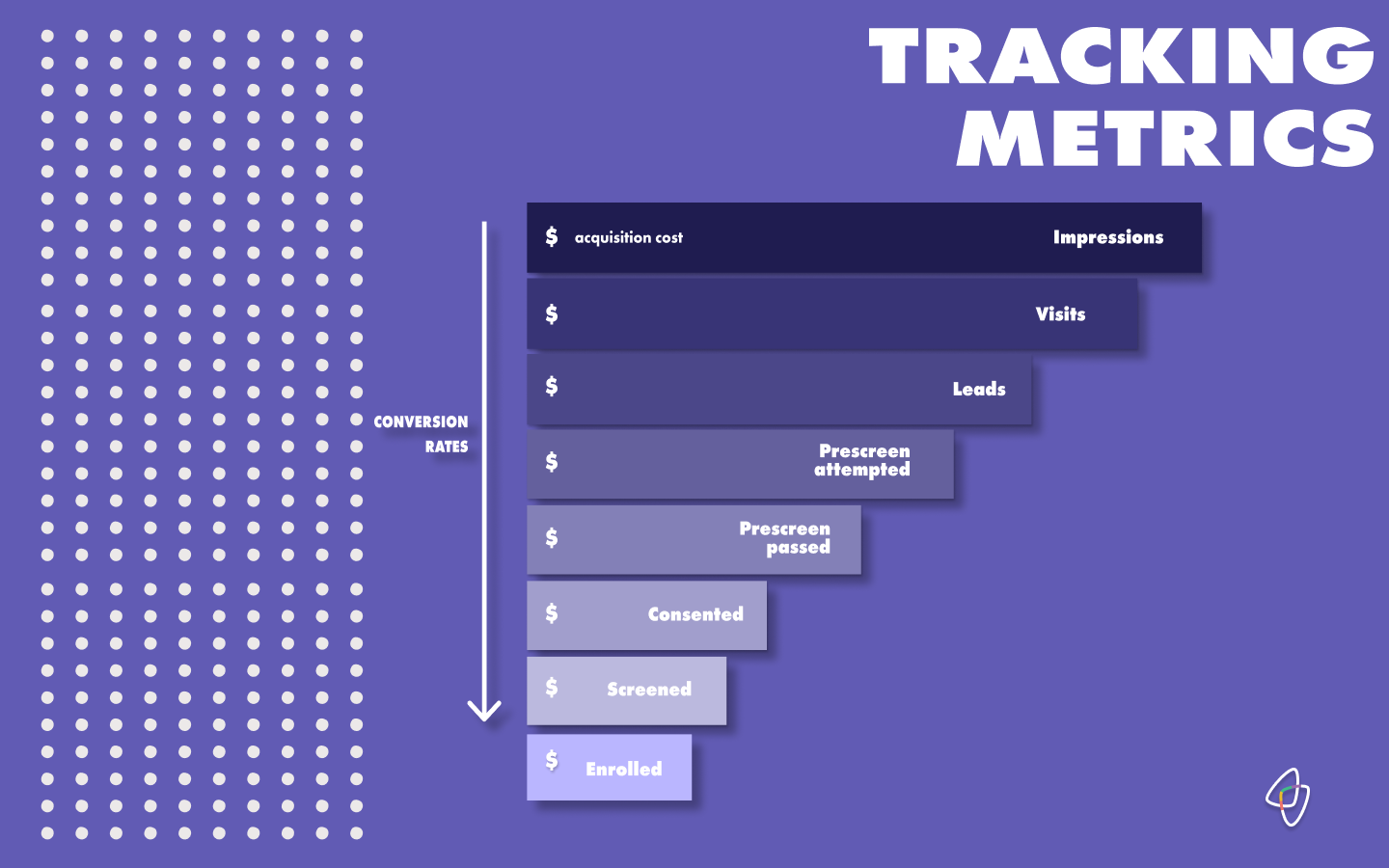
Just as one might track the analytics of their website, we encourage data management of average webpage visit length, daily active users, and the ratio of daily active users over monthly actives (among many other figures).
By thinking of patients as the “user”, we measure every single user’s flow through the funnel and are able to reduce the cost and time to bring that user through each step of the study process.
It should be common practice for teams to run a retrospective on the data collected from a recently-enrolled study to answer questions like “How many referred patients ended up enrolling” or “What factors were common among the reasons that unenrolled applicants chose not to proceed?”
However, it is equally important to run continuous analysis on a study’s recruitment funnel, even while recruitment efforts are ongoing.
Different channels may deliver different mindsets, wants, needs, or medical histories. Segmenting your candidates based on their source can help you respond to these different needs and desires of potential study candidates. Measuring heatmaps (heavy traffic) and visit duration (how long a user stays on a page, or bounce rate (how quickly someone leaves the site), you can refine and fix any drop offs. If a pre-screener is being administered on the website you can also take a look at where candidates are failing to complete the survey.
Unfortunately, the limitations of gathering data via a clinicaltrials.gov listing or from certain third-party landing pages can inhibit your ability to assess and refine gaps. Instead, your study team can maximize its chances at recruitment success by choosing to work with vendors who not only can create captivating landing pages but can also provide detailed performance data on a real-time basis.
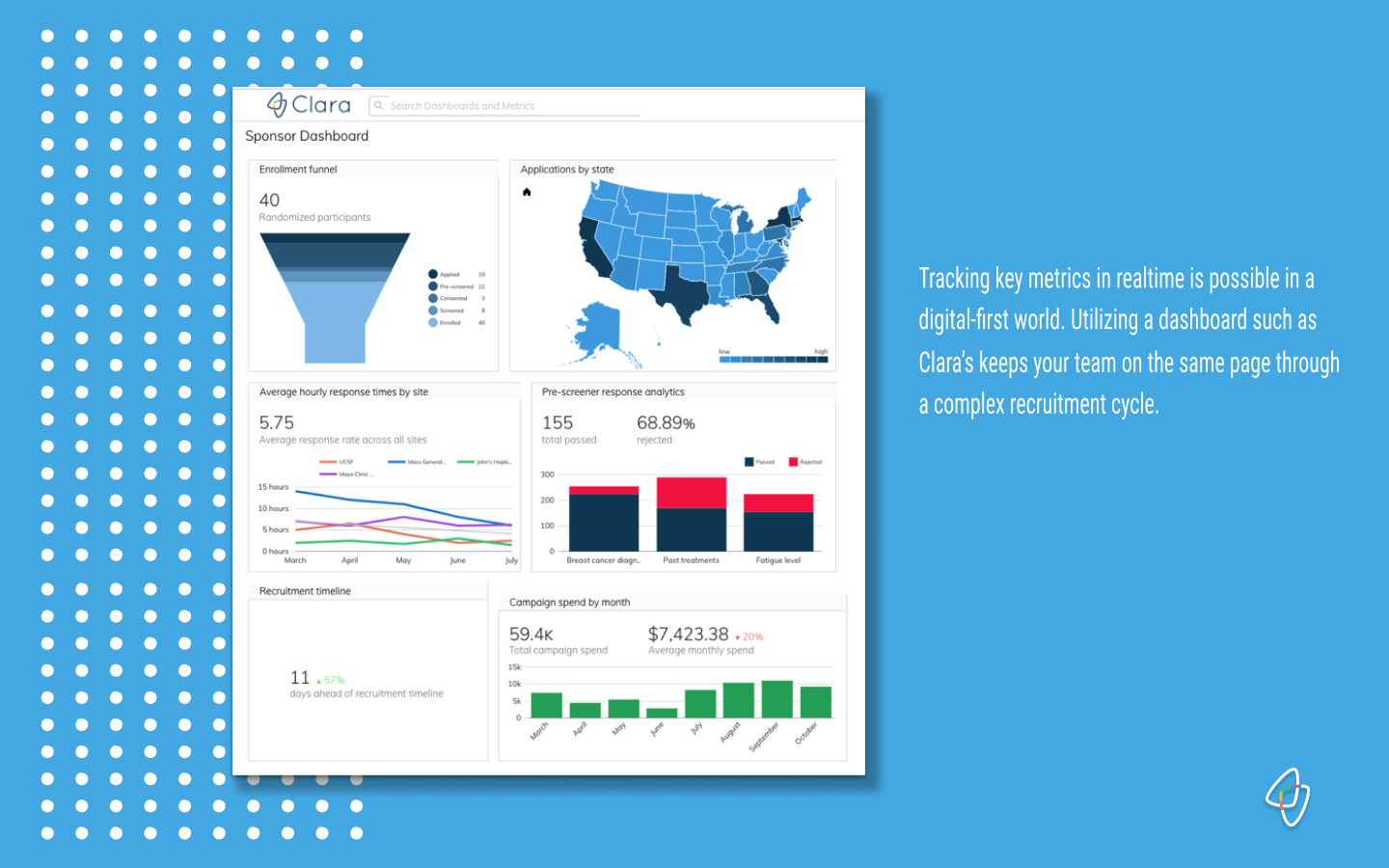
Clara Health client analytics dashboards are custom built according to the key metrics for that particular study. Each dashboard represents a 24/7 live view of recruitment and retention performance, and can be constantly updated to adapt to the needs of any trial.
To read about our proposal to a better solution, Read Ahead.
